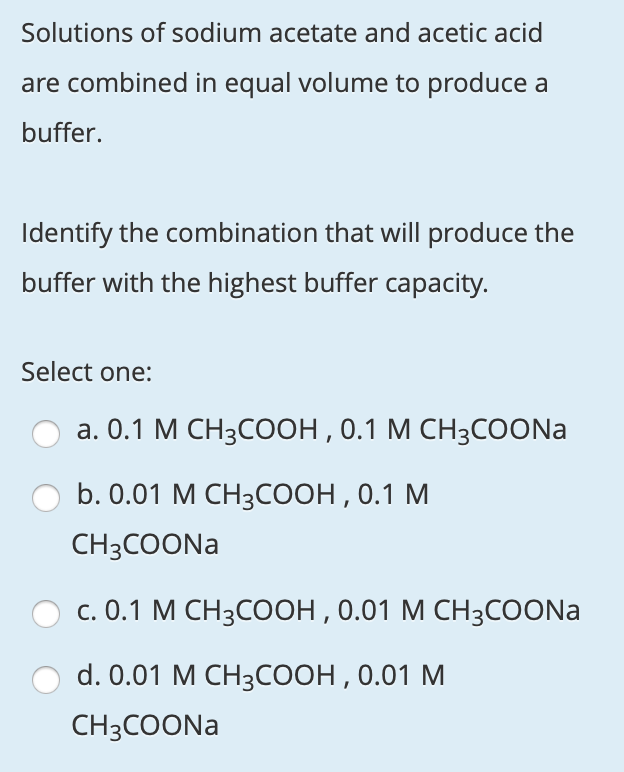
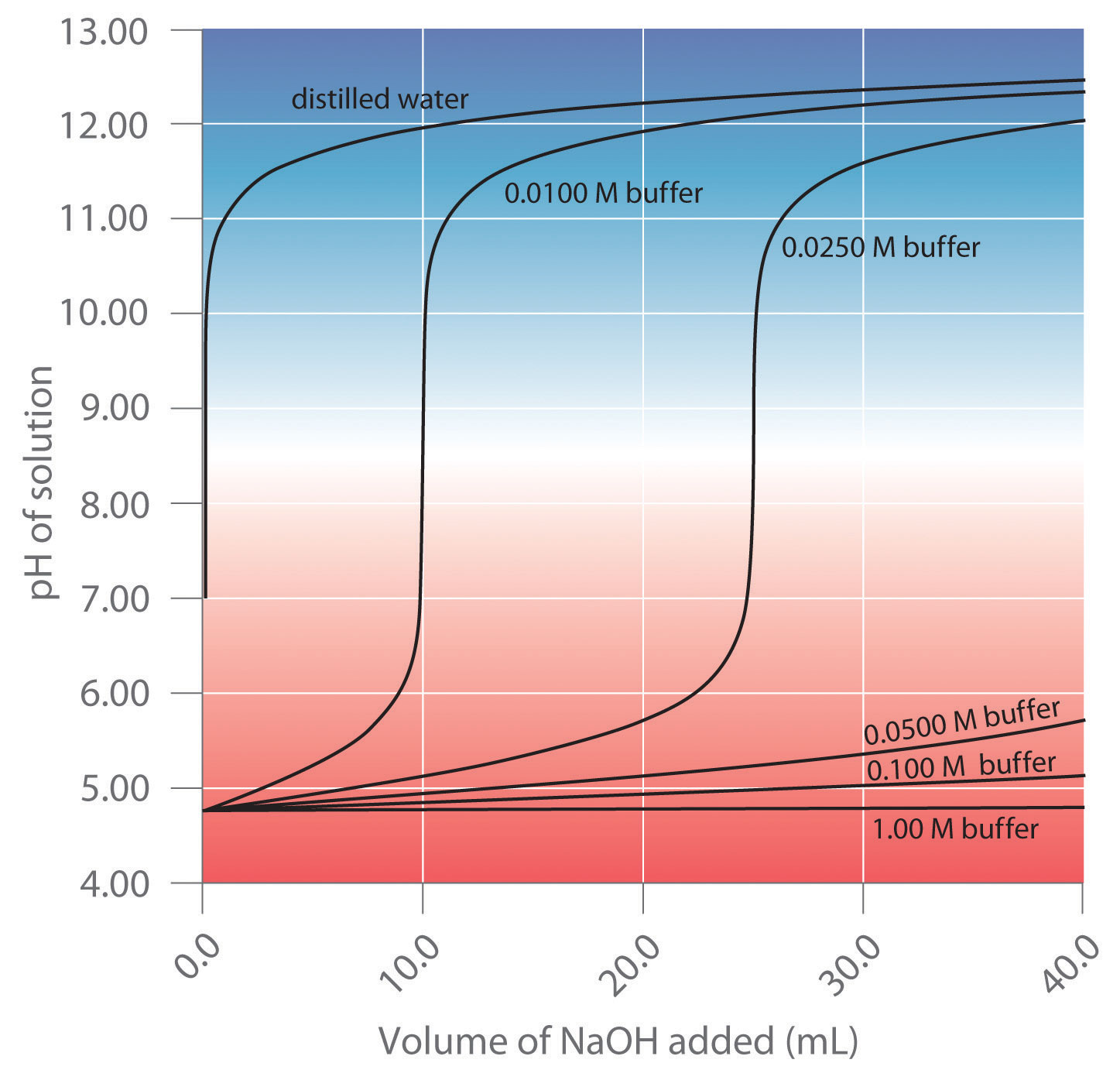
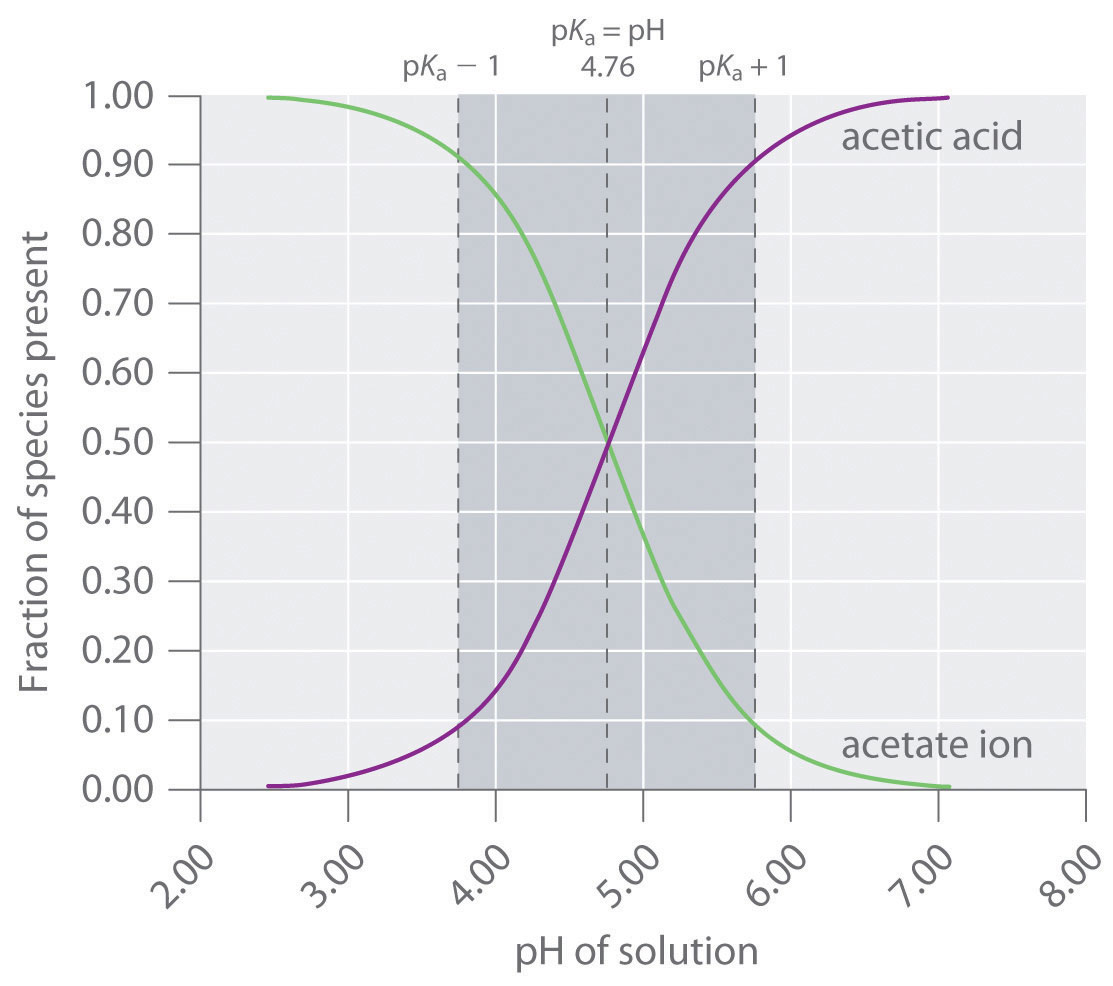
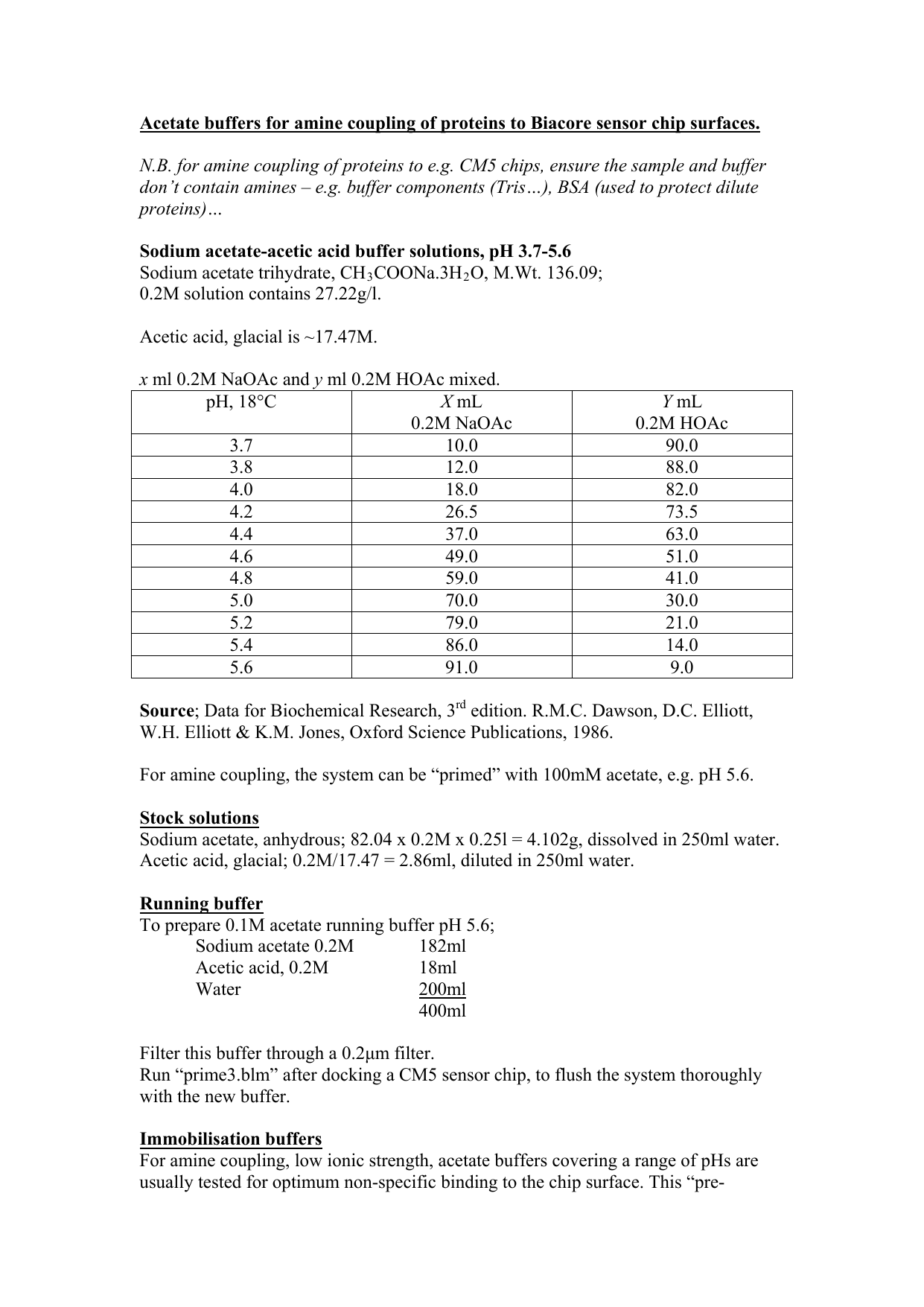
OneClass: A buffer contains significant amounts of acetic acid and sodium acetate. Write an equation ...

Chapter 17 Acid-Base & Solubility Equilibria The Common Ion Effect 17.2 Buffer Solutions 17.3Acid-Base Titrations (omitted) 17.4Solubility Equilibria. - ppt download

SOLVED: buffer solution is made from acetic acid, HCzH;Oz, and sodium acetate, NaCzH;Oz: Suppose small amount of hydrochloric What is the net ionic equation for the reaction that occurs when acid is
How to prepare 1oo ml of 0.200 M acetate buffer at pH 5.00 starting with pure liquid acetic acid and solutions containing 3M HCl and 3M NaOH - Quora

Preparation of acetate buffers using Sodium acetate and acetic acid using the Henderson-Hasselbach - Studocu

BUFFERS Mixture of an acid and its conjugate base. Buffer solution resists change in pH when acids or bases are added or when dilution occurs. Mix: A. - ppt download

SOLVED: Question 12 (1 point) What reaction occurs as HCl solution is added to a buffer solution containing equal concentration of acetic acid, CH3COOH; and sodium acetate, CH3COONa? CHzcoo CHzCOoH CHzCOOH Ht